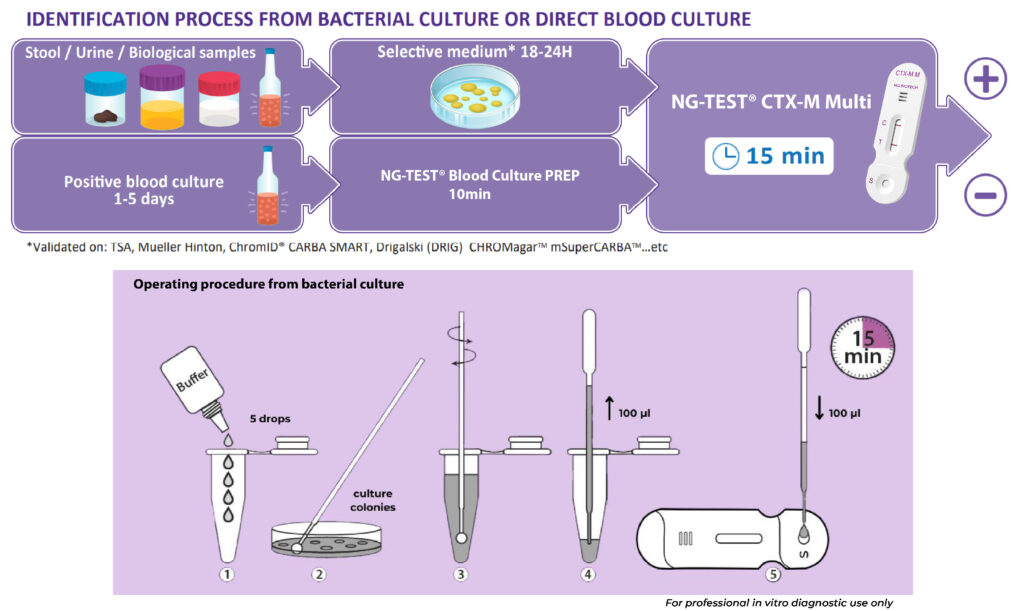
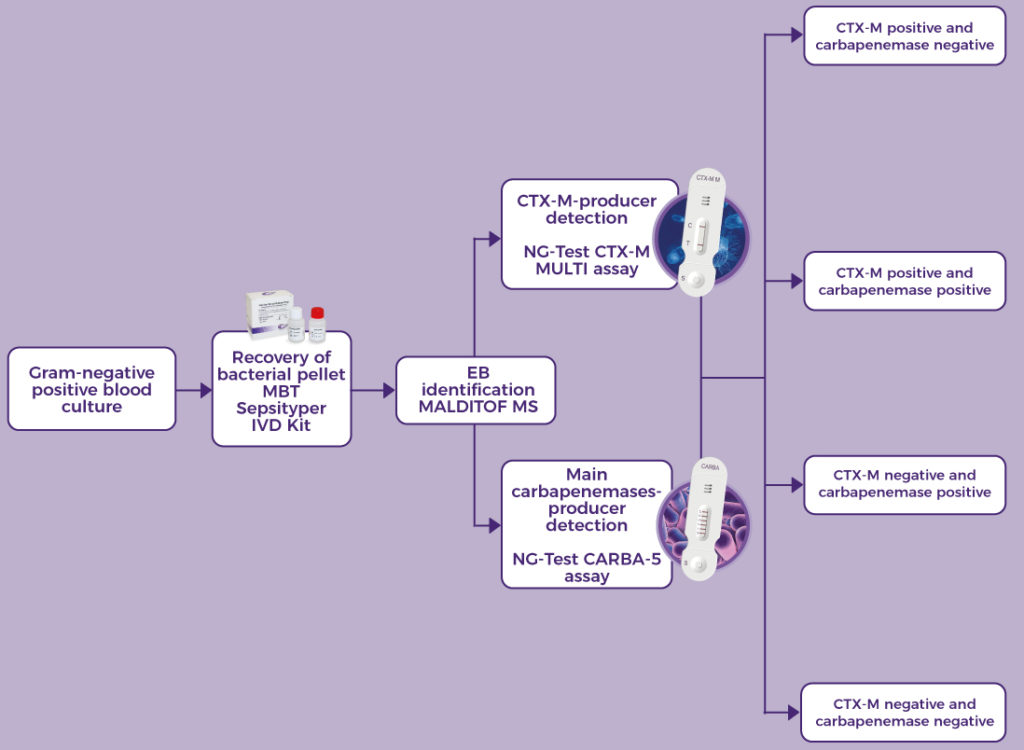
NG-TEST® CTX-M Multi is a rapid in vitro diagnostic assay, for professional use only. It is a visual multiplex immunochromatographic (lateral flow) qualitative assay for the detection of the five CTX-M groups 1, 2, 8, 9 and 25 from extended-spectrum β-lactamases (ESBLs) pure bacterial colonies of Enterobacterales (including Escherichia coli and Klebsiella pneumoniae) in healthcare settings.
The use of NG-TEST® CTX-M Multi in the laboratory provides information to inform appropriate antimicrobial therapeutic choices and support prompt infection control decision making and interventions.
The NG-TEST® CTX-M Multi can be easily implemented in any laboratory setting with minimal training.
It requires minimal hands-on time, the preparation protocol is simple and no instrumentation is required. NG-TEST® CTX-M Multi can also be performed directly from Positive Blood Culture samples, which can support treatment decisions about 1-2 days faster than current methods allow today.