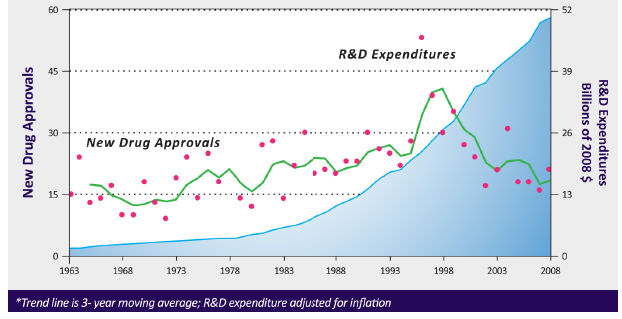
New drug approvals are not keeping pace with rising R&D spending.
Pharmaceutical companies are looking for ways to reduce costs and there is a positive receptivity to decentralized solutions.
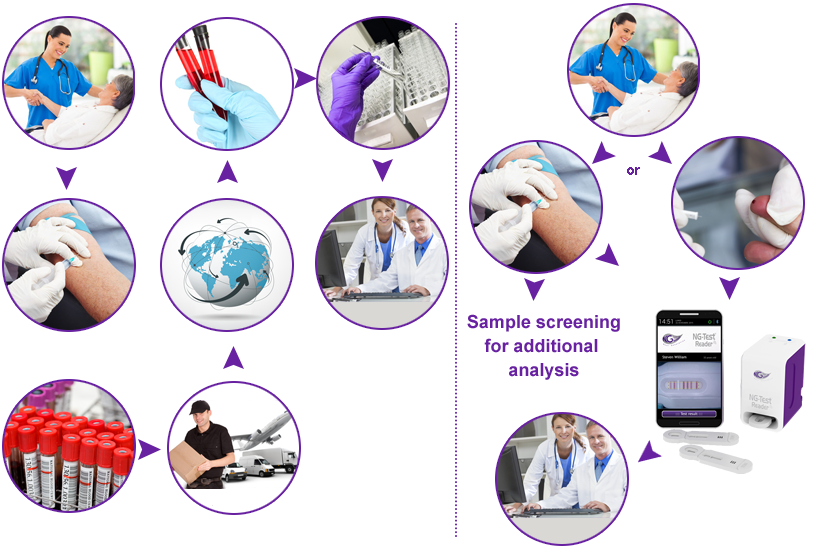
Central laboratory processes usually generate high collateral costs in biological sample shipping, sample and data management as well as in time to get results. At NG Biotech, we propose you to significantly Save Costs with Point of Care Testing processes which eliminate unnecessary sample shipping and management steps while getting results in Real Time.
Take a step ahead while developing a new drug and contact us for a contract development enquiry. We will meet your specific needs in new companion diagnostics with our Quantitative platform, Fast to develop and Cost effective Point of Care solutions.
Being experts of the Point of Care diagnostics market, in a partnership with Blinded Diagnostics, we also support planning and administering cost-effective same day diagnostic testing solutions for pharmaceutical clinical trials.